This post is a continuation of the series of articles aimed at providing some degree of in-depth understanding of various water treatment processes that may be considered for recycling applications. The topic this time is activated carbon treatment. Consistent with previous such posts, I have provided a couple of references at the end for those who may be inspired to research the topic further (or check that I’m not making it up as I go along!).
Activated carbon is a form of carbon usually derived from charcoal. The term ‘activated’ refers to the way the carbon has been prepared to enhance its ability to physically ‘adsorb’ chemicals to its surface. Adsorption is the accumulation of a dissolved chemical (solute) onto a solid surface.
An important property of activated carbon is its extremely high surface area. One gram (about a teaspoon full) of activated carbon can have a surface area of 400-2000 square metres. By comparison, a tennis court is about 260 square metres. A microscopic view of activated carbon reveals a complex web structure intermingled with trapped smaller particles. There are many nooks and crannies, which provide excellent conditions for adsorption of suitable chemicals.
Activated carbon is widely used in drinking water treatment for the removal of certain chemicals. The chemicals for which activated carbon is most effective are those which are relatively non-polar in their chemical structure.
A term commonly applied to describe chemicals is their degree of ‘lipophilicity’. Lipophilic chemicals are generally non-polar chemicals that are accumulated in lipids (fats and oils). The same properties that tend to make chemicals accumulate in lipids also tend to make them well adsorbed to activated carbon. Therefore, lipophilic chemicals are chemicals that are generally well removed from water by activated carbon treatment.
The most common applications of activated carbon for water treatment are known as granular activated carbon (GAC) and powdered activated carbon (PAC). These terms simply refer to the physical form (particle size) in which the activated carbon is applied. Smaller particle sizes (PAC) tend to have higher surface areas while large particle sizes (GAC) tend to be more easily separated from the water subsequent to treatment. PAC is often used by direct addition to water with mixing and then separated by gravity and/or filtration. Alternatively, GAC is more commonly used as filtration media with the water being percolated through it.
PAC and GAC are effective for the removal of a diverse range of lipophilic organic compounds as well as well as some relatively lipophilic inorganic compounds such as nitrogen, sulphides and heavy metals. However, more polar compounds are relatively poorly removed by activated carbon adsorption.
The parameter most commonly used to describe how well a substance can be adsorbed to activated carbon is known as the Freundlich capacity factor. The Freundlich capacity factor is determined experimentally by testing various ratios of chemical concentration and activated carbon surface area in otherwise clean waters under controlled conditions. A high Freundlich capacity factor indicates that the chemical is very effectively adsorbed, while a low Freundlich capacity factor indicates that the chemical is poorly adsorbed.
The range of Freundlich capacity factors for potential water contaminants is extremely wide. For example, polychlorinated biphenyls have Freundlich capacity factors greater than 10,000 while N-nitrosodimethylamine (NDMA) has a Freundlich capacity factor of around 0.0001. Furthermore, specific mixtures of compounds in a raw water source will affect the adsorptive capacity for each compound.
In 2005, a group of researchers from Arizona State University, Northwestern University (Illinois) and Southern Nevada Water Authority published a paper investigating the fate of endocrine-disruptors, pharmaceuticals, and personal care products during simulated water treatment processes [1]. For this research, they collected raw drinking water supplies and artificially spiked in high concentrations of 62 different chemicals. These waters were then treated by a number of laboratory-scale water treatment processes including PAC. Addition of 5 milligrams per litre of PAC with a 4-hour contact time removed different compounds by between 10% to greater than 98%. Higher PAC dosages improved the removal of most compounds. This study confirmed that the removal effectiveness for specific chemicals could be reasonably well predicted based on their lipophilicity.
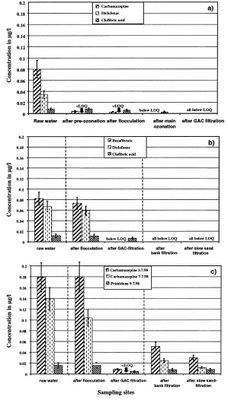
GAC has also been shown to be effective for the removal for some important organic chemical contaminants in water. For example, a group of researchers in Germany has investigated the removal of selected pharmaceuticals during a range of drinking water treatment processes [2]. This study revealed GAC filtration to be an effective method for removing most of the studied compounds. The image below shows the relative concentrations of some of these compounds that were actually measured in raw drinking water sources (that is, they were not artificially spiked in). It can be seen that GAC treatment, in combination with other conventional treatment processes, significantly removed most of the pharmaceuticals. In a number of cases, remaining concentrations were reported to be below the limit of quantitation (LOQ). This simply means that the concentrations were too low to be reliably measured.
These studies are consistent with the conventional understanding and application of activated carbon treatment processes. Like all water treatment processes, activated carbon is not a silver bullet and is not intended to be so. However, used as a component of a carefully selected suite of treatment processes, activated carbon has an important role to play in water purification.
As always, I'd be grateful for your comments...
[1] Westerhoff, P., Yoon, Y., Snyder, S. and Wert, E. (2005) Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Journal: Environmental Science and Technology, Volume: 39, Issue: 17, Pages: 6649-6663.
[2] Ternes, T. A., Meisenheimer, M., McDowell, D., Sacher, F., Brauch, H.-J., Haist-Gulde, B., Preuss, G., Wilme, U. and Zullei-Seibert, N. (2002) Removal of pharmaceuticals during drinking water treatment. Journal: Environmental Science and Technology, Volume: 36, Issue: 17, Pages: 3855-3863.
Since I stole the nice activated carbon image from another website, I suppose it would be polite to point you to their site as an example of a company that supplies GAC and PAC for a wide range of applications...not just drinking water treatment.
"An unemotional and rational discussion of the facts as best that they can be scientifically supported". The aim of this blog is to make information available to concerned or interested members of the community.

- Stuart Khan
- Sydney, New South Wales, Australia
- I am a water researcher in an Environmental Engineering Department. Topics on which I work include: • Analysis of trace chemical contaminants in aquatic environments • Analytical method development (gas chromatography, mass spectrometry, etc) • Environmental and public health risk assessment of chemicals in the environment • Environmental chemical modelling techniques • Evaluation of water and wastewater treatment processes in terms of chemical fate • Water recycling practices in Australia and internationally • Guidelines for the management of chemicals in water.

5 comments:
Stuart, just wondering if you have any information on the use of GAC for the removal of endocrine disruptors, and any idea of the contact time required to do so? I understand it's a fairly recent topic and am interested to find out more. Ta!
Hello Anonymous,
Yes, it is a relatively recent topic, but there is much current research activity. If you have access to a library with a good scientific journal collection, I suggest you take a look at the following references...
I hope you find this to be of some help.
Stuart
Ifelebuegu AO, Lester JN, Churchley J, et al.
Removal of an endocrine disrupting chemical (17 alpha-ethinyloestradiol) from wastewater effluent by activated carbon adsorption: Effects of activated carbon type and competitive adsorption
ENVIRONMENTAL TECHNOLOGY 27 (12): 1343-1349 DEC 2006
Snyder SA, Adham S, Redding AM, et al.
Role of membranes and activated carbon in the removal of endocrine disruptors and pharmaceuticals
DESALINATION 202 (1-3): 156-181 JAN 5 2007
Bodzek M, Dudziak M
Elimination of steroidal sex hormones by conventional water treatment and membrane processes
DESALINATION 198 (1-3): 24-32 OCT 30 2006
Choi KJ, Kim SG, Kim CW, et al.
Removal efficiencies of endocrine disrupting chemicals by coagulation/flocculation, ozonation, powdered/granular activated carbon adsorption, and chlorination
KOREAN JOURNAL OF CHEMICAL ENGINEERING 23 (3): 399-408 MAY 2006
Zhang YP, Zhou JL
Removal of estrone and 17 beta-estradiol from water by adsorption
WATER RESEARCH 39 (16): 3991-4003 OCT 2005
Choi KJ, Kim SG, Kim CW, et al.
Effects of activated carbon types and service life on removal of endocrine disrupting chemicals: amitrol, nonylphenol, and bisphenol-A
CHEMOSPHERE 58 (11): 1535-1545 MAR 2005
Fuerhacker M, Durauer A, Jungbauer A
Adsorption isotherms of 17 beta-estradiol on granular activated carbon (GAC)
CHEMOSPHERE 44 (7): 1573-1579 SEP 2001
Am doing an end of semester project on the best way to treat pharmaceuticals in water and find you article very useful,what are your views on ozonation,do you think it is a better option than nanofiltration and more cost effective.
Hill
Hi,I'm Trish.
I would like to have your comments regarding the use of powdered activated carbon in the sludge process.I am actually doing my degree project having the above title.What are your views concerning the cost of PACT systems.Can PACT systems be applied to a small country??Thanks.
Hi I am Jimmy from Malaysia, a PhD research student in activated carbon. We have successfully producing activated carbon using palm shell, a agri-waste vastly found. Our AC is with high fraction of mesopores (larger pores 2-50nm), surface area of 3,000 m2/g, which is great for macromolecules adsorption. Just wonder if it is suitable for PACT application. Is so, what are the potential pollutant we could target. Thanks
Post a Comment