1. Size exclusion
2. Hydrophobic adsorption
3. Electrostatic repulsion
I’d like to examine this topic in a little more detail and to do so, I will introduce something known as a ‘membrane rejection diagram’. Some of this post uses some unavoidable scientific terminology...I apologise for that but don’t worry: you don’t need to be familiar with every single term in order to gain an understanding of the general concept presented.
Chris Bellona is a PhD student working with Associate Professor Jörg Drewes at the Colorado School of Mines. Bellona has spent his PhD studies closely examining which chemicals are able to pass through different membranes and which ones are rejected by the membranes. By understanding the three rejection mechanisms listed above, Bellona was able to categorise different chemicals according to their chemical properties that would determine how effectively they would be rejected by a specific membrane. The important molecular properties identified by Bellona are:
• Molecular size: The size of a molecule is often approximated by reference to its molecular weight (MW), but can be more accurately described in terms of its molecular diameter and molecular width (MWd).
• Electrostatic properties: The electrical charge of a molecule is related to how acidic it is. This is commonly described by an acid dissociation constant (pKa) and its relationship to the overall acidity of the water (pH).
• Polarity or hydrophobicity: The ‘polarity’ of a molecule determines whether it is generally very soluble in water or would prefer to partition to non-water phases. Molecules that tend to partition away from water are said to be ‘hydrophobic’. The degree of hydrophobicity is commonly described by an ‘octanol-water partitioning coefficient’ (Log Kow).
The most fundamental of the rejection mechanisms is size exclusion. This is a sieving process for which molecular size or geometry prevents large molecules from passing through the dense molecular structure presented by the active surface of the membrane. Depending on the particular membrane being used, size exclusion is believed to be the dominant retention mechanism for relatively large molecules such as surfactants, hormones, most pharmaceuticals, proteins and other molecules with MW greater than 200 atomic mass units.
However, commercial membranes vary in terms of their ability to reject molecules by size exclusion. Their ability to do so is often described by the membrane’s Molecular Weight Cut-Off (MWCO). This is the manufacture's rating of the membrane's ability to reject an uncharged dextran (sugar) based on molecular weight. Membranes with a low MWCO are commonly referred to as ‘tight’ membranes compared to those with a higher MWCO, referred to as ‘loose’ membranes.
Experiments with looser membranes (nanofiltration, ultrafiltration and microfiltration), have revealed that under some conditions, some chemicals are prevented from permeating the membrane due largely to adsorption to the membrane surface. This adsorption is believed to be due to hydrophobic interactions between relatively non-polar molecules and membranes. Such adsorptive removal may be less reliable than removal based purely on size exclusion since variations in solution pH lead to variations in hydrophobicity, and possible saturation of adsorption sites may limit total adsorption capacity if the membranes are not routinely cleaned.
Some modern reverse osmosis membranes have been designed with chemical functional groups attached to the membrane surface. These functional groups can be negatively charged, thus they repel molecules that are also negatively charged away from the membrane. They are designed to do this since it requires much less energy to reject molecules by this mechanism than it would to rely on size-exclusion alone.
By considering the combination of the properties of a particular contaminant (MW, pKa, Log Kow, MWidth), the water solution (pH) and the particular membrane (MWCO, surface charge), general rejection behaviour can be estimated by the following rejection diagram developed by Bellona and Drewes. Click on the image to enlarge for a better view.
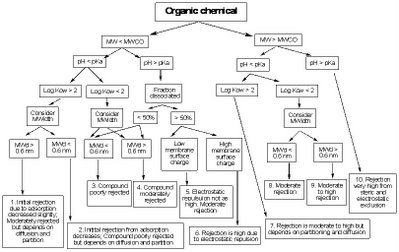
Some time ago, I went to the trouble of setting up an Excel spreadsheet that allows me to enter the properties of a membrane and a molecule and determine which of the above ten rejection categories the molecule should fall into (call me a geek, I can take it). I ran a large number of chemicals through the spreadsheet and the results were very pleasing. I found that the predicted behaviours matched reported experimental observations very well.
For example, the rejection diagram predicted that the pharmaceuticals acetylsalicylic acid (aspirin), clofibric acid, diclofenac, gemfibrozil, ibuprofen, ketoprofen, naproxen, and propyphenazone would all fall into category 10 for a membrane with MWCO 100. This predicted very high rejection is consistent with published experimental observations. Alternatively, some compounds such as 1,4-dioxane, 1,2-dichloroethane, dichloromethane and nitrosodimethylamine (NDMA) fell into category 3 indicating that they were predicted to be poorly rejected. Again this is very consistent with observed behaviour and (in the case of 1,4-dioxane and NDMA) precisely the reason that advanced oxidation was added to indirect potable water recycling schemes in California.
Of course, back in the real world things are not quite as simple as this neat rejection diagram suggests. Other important factors that contribute to rejection include the type of spacer material used to form the membrane feed channels and the system operating conditions including pressure, flow rate of water across the membrane and precise water chemistry. For this reason rejection data determined in simple lab scale experiments should be interpreted cautiously before drawing conclusions on full scale plant performance because the conditions under which the membranes operate will be different.
During normal operation, membranes are prone to fouling by the build-up of precipitated chemicals retained by them or by the growth of biomass. Fouling can lead to significant changes in membrane surface properties and thus in the way in which the membranes interact with water and dissolved contaminants. In many cases, fouling is regarded as a hindrance since it decreases membrane porosity and thus requires elevated pressures to maintain the flow of water across the membrane.
However, recent investigations reveal that fouling can also lead to improved rejection of many solutes. This observation is believed to be due to a number of factors including partial pore-blocking (thus effectively reducing the MWCO). Other factors may include increased negative surface charge leading to increased electrostatic rejection of ionic species; and increased adsorptive capacity for hydrophobic chemicals.
Most previous studies reporting relationships between physical-chemical properties of solutes and membrane interactions have been conducted using unfouled ‘virgin’ membranes and thus their conclusions are unlikely to be quantitatively transferable to full-scale systems subjected to long-term operation. Indeed, many such studies were used in the derivation of the rejection diagram by Bellona and this must be seen as a limitation to its current usefulness.
More details regarding Bellona’s membrane rejection diagram can be found in the following publication:
Bellona, C., Drewes, J. E., Xu, P. and Amy, G. (2004) Factors affecting the rejection of organic solutes during NF/RO treatment--a literature review. Water Research, Volume 38, Issue 12, Pages 2795-2809.

17 comments:
Hi Stuart,
Are you still at this, thought you would have given up, since the support for this subject has dropped to very low.
I don't think explaining what chemicals can get through and what can’t is going to help. It's just a simple matter that people are not prepared to risk what water they have now to new technology, in the event of an accident.
Don't you realize the outcome if the dams in SEQ happen to fall under an accident due to recycled water, regardless of the safe guards in place?
You’re looking at over 1Million people without water. That it self is far too big of a risk to take. If people like you (Thorley, Beattie, Howard) are prepared to take that risk, then it's us people who suffer because of your stupidity.
There is NO proven facts that dumping this so called purified water back into the dams will have no effect.
If it does, it will be way too late to do anything about it. I personally would take measures in preventing such a technology to be introduced into our already very low water supplies, it us who have to live with your decision and some idiot who thinks only for him/her self.
I no longer see this debate about recycled water, it's now a political agenda and a money grabbing scheme, again with our lives as the bait!
We have more sources of water then just recycled sewerage, but you don't seem to be pushing those ideas out there, no, so you have an agenda here as well.
It's high time you and people like you start to realize we need to source natural water NOW! Not in a few years.
You need to do your maths, sit down and think about for a moment, recycled sewerage will only claim a small portion back, which is no where near enough to sustain a water supply in time of harsh drought, so how can you sit there an poor all this information down our throat without stating the obvious.... Recycled water will NOT sustain a cities water supply, only prolong the inevitable.
Even if the Toowoomba was yes, it wasn’t going to be introduced until at least 2010, by then the dams would have run dry. So it’s no different here and it will be no different in Brisbane or anywhere else for that matter. Australians will want this technology tested and PROVEN to be safe before subjecting it to our water supply.
The first I hear if any recycled sewerage plant being built, I will be the first to chain myself to the trees and rocks, and if I have concrete my feet into the ground.
I’m sick of hearing about this subject, and the Toowoomba referendum should have been the vote to say YES or NO Australia wide.
You should look at better ways of saving our current water supply instead of worrying about recycled shit!
Thanks Nijel,
I appreciate your comments. I would like to point out (for the umpteenth time) that it is not up to me how Brisbane (or Toowoomba or anywhere else) manages its water. Those decisions are up to people like you and the representatives that you elect. My role (if any) is to try to provide accurate information which, quite honestly, some people seem to be crying out for.
If you are sick of hearing about this subject, then you are probably reading the wrong blog. Nonetheless, you are most welcome to hang around if you can stand it and your feedback is appreciated.
Stuart, have you come across any studies which assessed the impacts/effects recycled water has on mammals and fish after long term exposure (over a number of generations) and ingestion? I am aware if the study carried out in Singapore, but I not sure if it has been completed. There was also a similar study done in Denver I think, but back in the '80's.
If you have come across such work, I think that a posting on this topic would be interesting and would serve as an evidence of the safety of reclaimed, recycled water from an AWTP over the long term, which is what some sceptics have been asking for.
Just a future posting thought...
G’day Njta,
Yes, I think you are correct in identifying that actual health impacts are the real issue, rather than getting too wrapped up in individual chemicals (but hey, that’s what us water chemists do!).
The answer to your question is a very big YES. I have recently completed an extensive review of the types of studies that you have described. There is plenty of good information out there, particularly from studies undertaken in California, Florida and (as you say) Colorado.
However, this review document was commissioned by the Local Government Association of Queensland so it is now their property. Its main purpose was to make the information available to their members (mayors and councillors) so that they could make an informed opinion regarding the safety of recycled water. However, I understand that they plan to publish it and make it publicly available within the next fortnight. So rather than steal their thunder, I’ll wait for them to publish it and then I will follow it up with a blog post soon after, -which will obviously provide people with the opportunity for feedback.
Thanks for the advice and I would be grateful for any other suggestions regarding specific topics of interest.
What a load of propaganda that document is sure to be!!
Thanks for your informed opinion Anonymous. However, I can’t help thinking that your argument might be more convincing if you would wait until you can at least pretend to have read it before passing such harsh judgement.
I look forward to reading it when the LGAQ releases it.
Stuart, we who have been through this debate before are ready and wait with baited breath the information our politicians will get to read when this document is released.
We have our own experts and I say " GAMES ON!"
Hello Anonymous,
I do find it slightly disconcerting that a small number of people seem to consider this as some kind of game to win or lose. I hope you will keep in mind that there are some very serious issues at stake and a large number of people involved. Consideration of this issue, and the debate that will surely come with it, should not be about scoring points, but about working cooperatively towards identifying solutions.
Stuart,
I would have to burry my head in the ground to not hear about recycled sewerage.
I do live in the town that had the first vote on it. I hear it all the time.
I am more interested in hearing logical facts about this technology. You can’t just dump into the water supply and prey that we got everything right.
As someone mentioned, it should be tested on a NON-DRINKING supply dam, and only after a period of time be used to help lower the use of dams, not replenish them.
All I have seen so far from the people who want this, is a quick nasty solution, dump it in the dam and hope it works out fine, worry about problems later. Just like they do when people get killed in a car accident and an intersection, wait for someone else to be killed and then another before they do anything about it, a waste of life. The same goes for the recycled water, force them to drink it and worry about illnesses or dam contamination later
It would be best if this technology was tested on a dam not is use, or a dam built specially to test the water. It’s too much of a risk to dump back into drinking supplies, do you agree?
The technology may be safe it may not be, and if the people are prepared to test it on their drinking supply without preliminary tests, then I guess we have them to blame if anything does go wrong.
If you can’t agree we need to source water from all possible avenues, then you have a one track mind, and shouldn’t be supporting just recycled sewerage. Telling us what chemicals are trapped by the membranes isn’t going to support that the water is safe, and won’t cause any damage to dams. Water is more precious then gold at the moment; we can’t afford to risk it for anything.
Thanks Nijel,
I can now clearly see that your concerns are genuine (and perfectly sensible).
Actually, I do agree with you. I also would be somewhat concerned if (I presume we are talking about Brisbane) started to charge one of the city's major reservoirs with recycled water without extensive testing of the water first.
Its not necessary (or practical) to build a new dam to do this. You can test the water quality prior to going into a dam at the exit point of the advanced water treatment plant. Until the tests have been completed, the water could be used solely for industry, discharged to the environment (in a non drinking catchment area) or even just disposed of to the sewers.
In the case of Brisbane, they could either build a pilot-scale plant to undertake testing on or they could build the full-scale plant, but use it initially for non-potable purposes.
I really don't think anybody wants a 'quick nasty solution', but I do agree that there is a sense of urgency to address urban water supply shortages in a number of locations around Australia. I think it is warranted.
"I do find it slightly disconcerting that a small number of people seem to consider this as some kind of game to win or lose."
Toowoomba's mayor and her engineer sought fame and glory by trying to be the first city in Australia to force their residents to drink recycled sewage. They put their own self interest above the interests of their community, doing anything to try to get their way.
Beattie uses the March poll as a distraction from his political problems, particularly the Merri Rose committal hearing - which starts on the same day that Beattie will decide the recycled water poll question in parliament. He doesn't care how south east Qld votes.
Beattie and Bligh then propose zero testing for the recycled water - just get it into Wivenhoe asap.
Whether you like it or not it's a game to the politicians and their sidekicks.
Before criticising someone for calling game on, remember who started this game in the first place. It's a game to them.
PRK,
Yes, your suggestion that water recycling has become a political football to be used and abused by some politicians is undeniable. I saw it happen during the Toowoomba debate and expect to see it happen more in Brisbane. However, the poor behaviour of some of our politicians should not be an excuse for those of us who consider the issues serious to sink to their standards. I hope that we can aim a little higher than that.
The best way to resolve differences is by listening to people’s concerns and taking them seriously. Municipal water management has become a polarising issue in this country, which I think is a reflection of the urgency of the situation and the numerous drawbacks associated with all of the proposed solutions (including dams, pipelines, desalination and recycling). The only way that we will find satisfactory solutions is if we work to genuinely cooperate, rather than antagonise.
Just playing the game the way they're setting it up.
When politicians are prepared to engage in genuine consultation rather than a $10 million ad campaign and electoral blackmail, maybe it will be time to work in a co-operative manner.
I doubt if that will ever happen as they all seem hell bent on making us drink the stuff no matter what, for some strange reason!
Stuart you work in composing a membrane rejection diagram is a useful reference when explaining to the general public how due diligence is being applied to the use of reclaimed water. Non potable end use applications that go over and above the authority requirements by including RO in treatment process need to be able to communicate to the public the ways risk is managed. This is an important part of the engineering process and risk management process that alays concerns about reclaimed water use. Well done.
Post a Comment